Diabetes is a major health crisis. The CDC estimates that one-third of the population has diabetes, or is at risk of developing diabetes.
Neural Networks for Glucose Forecasting
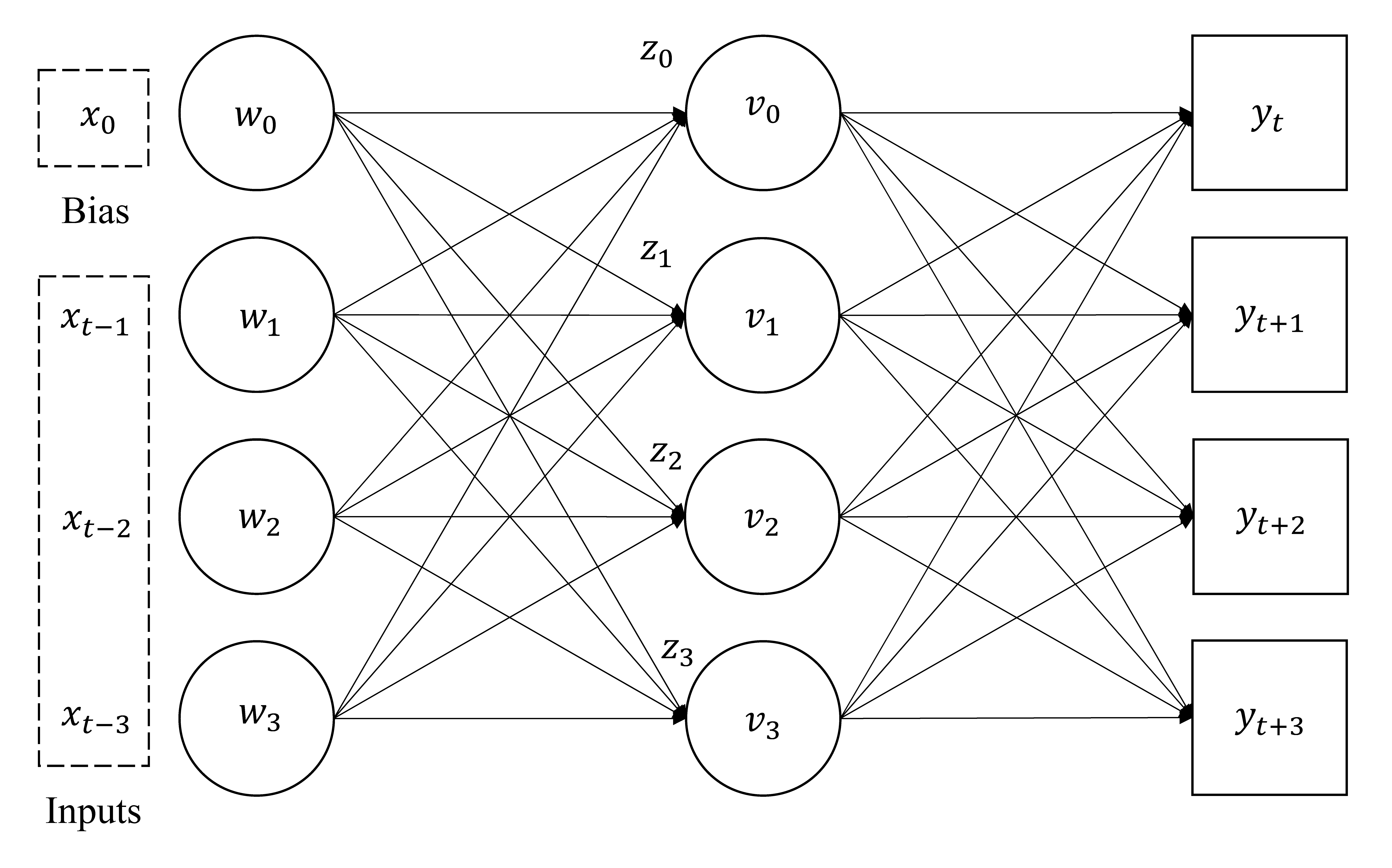
Patients with diabetes are unable to control their blood glucose (sugar) which can lead to serious long-term health issues such as cardiovascular disease. As a result, patients with diabetes need to routinely monitor their blood sugar to ensure it is within normal ranges. Traditionally, this was done by pricking a finger and directly measuring glucose using a handheld device. Modern advances in diabetes therapy has led to the increased use of Continuous Glucose Monitors (CGMs) which are small devices with tiny needles placed on the surface of the skin. CGMs monitor glucose continuously which gives both patients and providers significantly more data to use for treatment.
Cutting edge diabetes research is often concerned with forecasting future glucose values using CGM data since treatment can be better adjusted with advanced knowledge. One of the most popular forecasting methods is a neural network (NN) which utilizes previous CGM measurements to predict future glucose. Throughout my research I developed and tested over 10 unique NN structures for glucose forecasting.
Discovering the Effects of Sensor Location
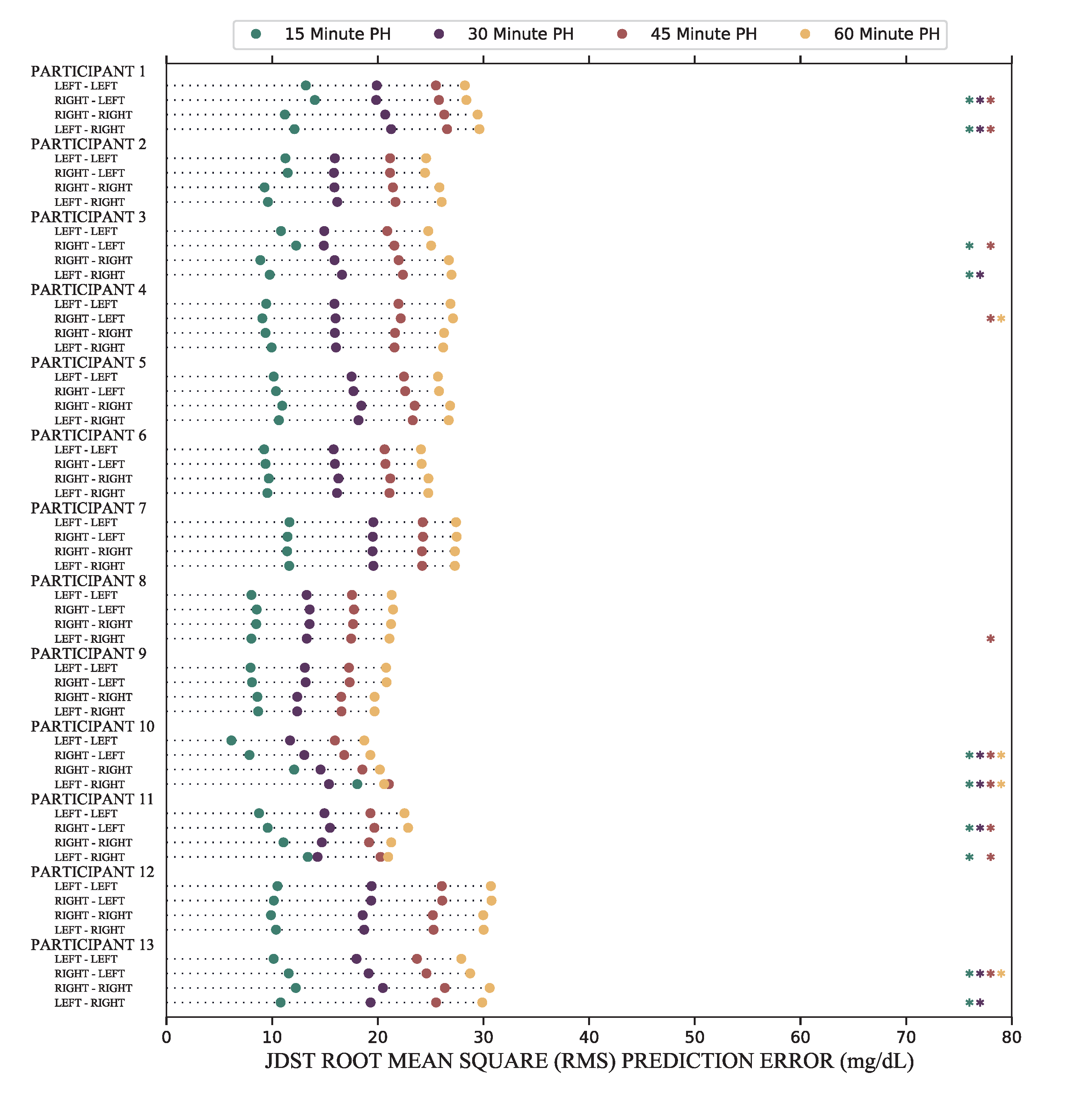
CGMs are designed to be placed on the back of either arm for up to 2 weeks, yet there has been little investigation into whether moving the sensor from one arm to the other can reduce the accuracy of glucose forecasts. Using a rigorous statistical testing procedure with data collected from patients with diabetes, I constructed a simple, feedforward neural network to test whether forecast error increases if the CGM is moved from the left to the right arm (and vice versa).
Results showed that prediction algorithms which performed well on CGMs only placed on one arm did not always perform as well when the CGM was moved to the other arm. In the graph, each asterisk (*) represents an instance where the forecast error increased due to a change in CGM location. This novel observation was published in the Journal of Diabetes Science and Technology.
Mitigating the Effects of CGM Location
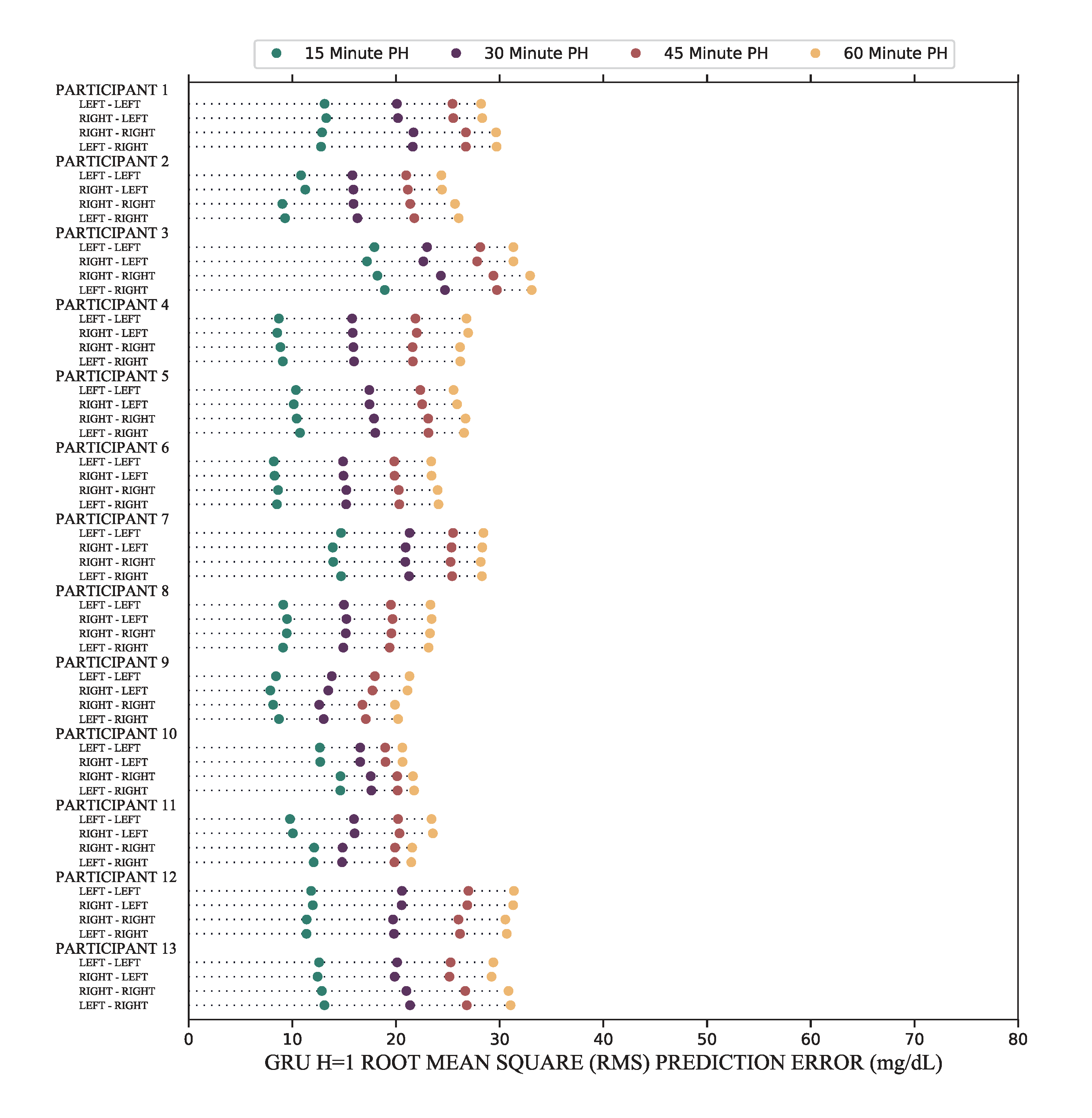
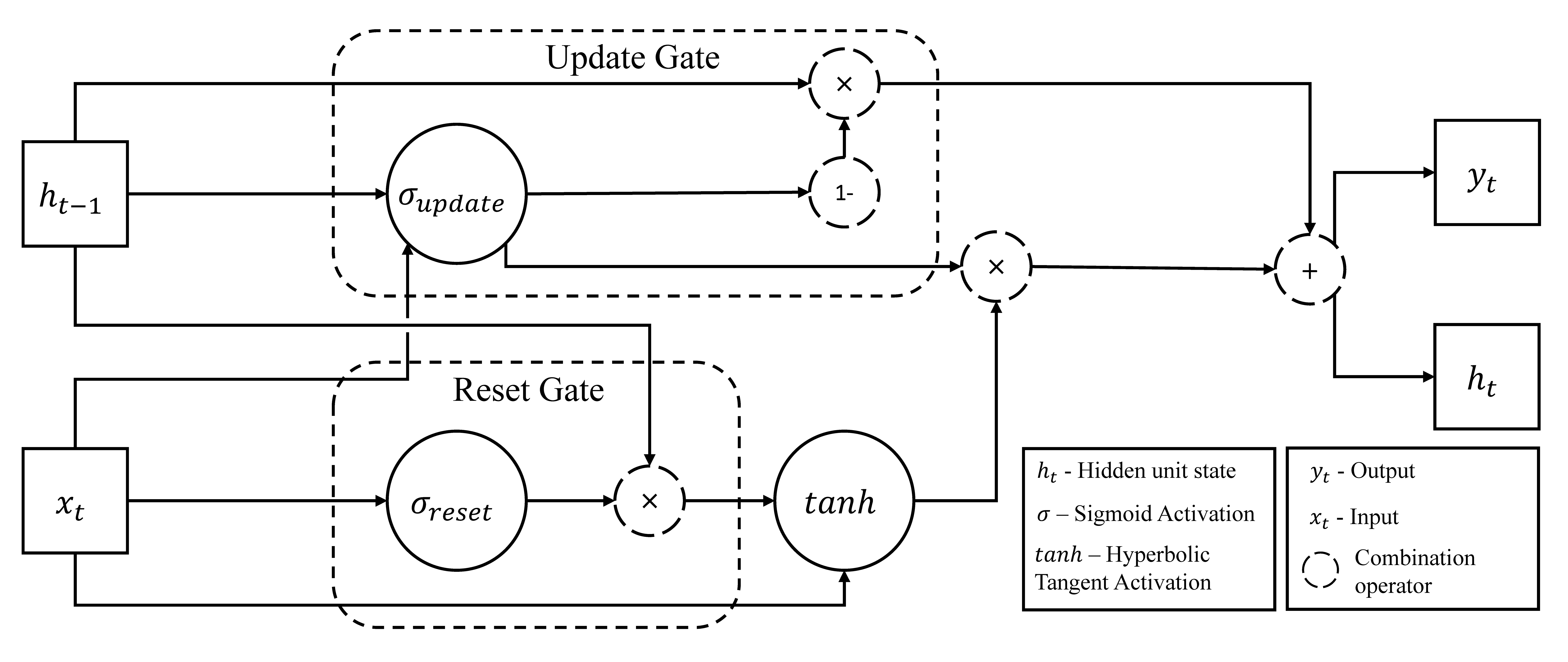
Clearly, feedforward NNs are not robust to changes in sensor location. These results motivated investigation of new methods for forecasting. My final design was a neural network with gated recurrent units (GRUs). These are a relatively new type of network which use results of previous forecasts to improve future forecasts. In this way, the network better manages both short- and long-term dependency.
I used the same test method to determine whether error increases when CGMs are moved between the right and the left arms. The results showed that GRUs are able to mitigate the error due to CGM location changes which makes them a more robust choice for glucose forecasting. In the graph, there are no instances of statistically significant increases in error due to these changes. These results were published as a companion paper in the Journal of Diabetes Science and Technology.
Improving Neural Network Training Time
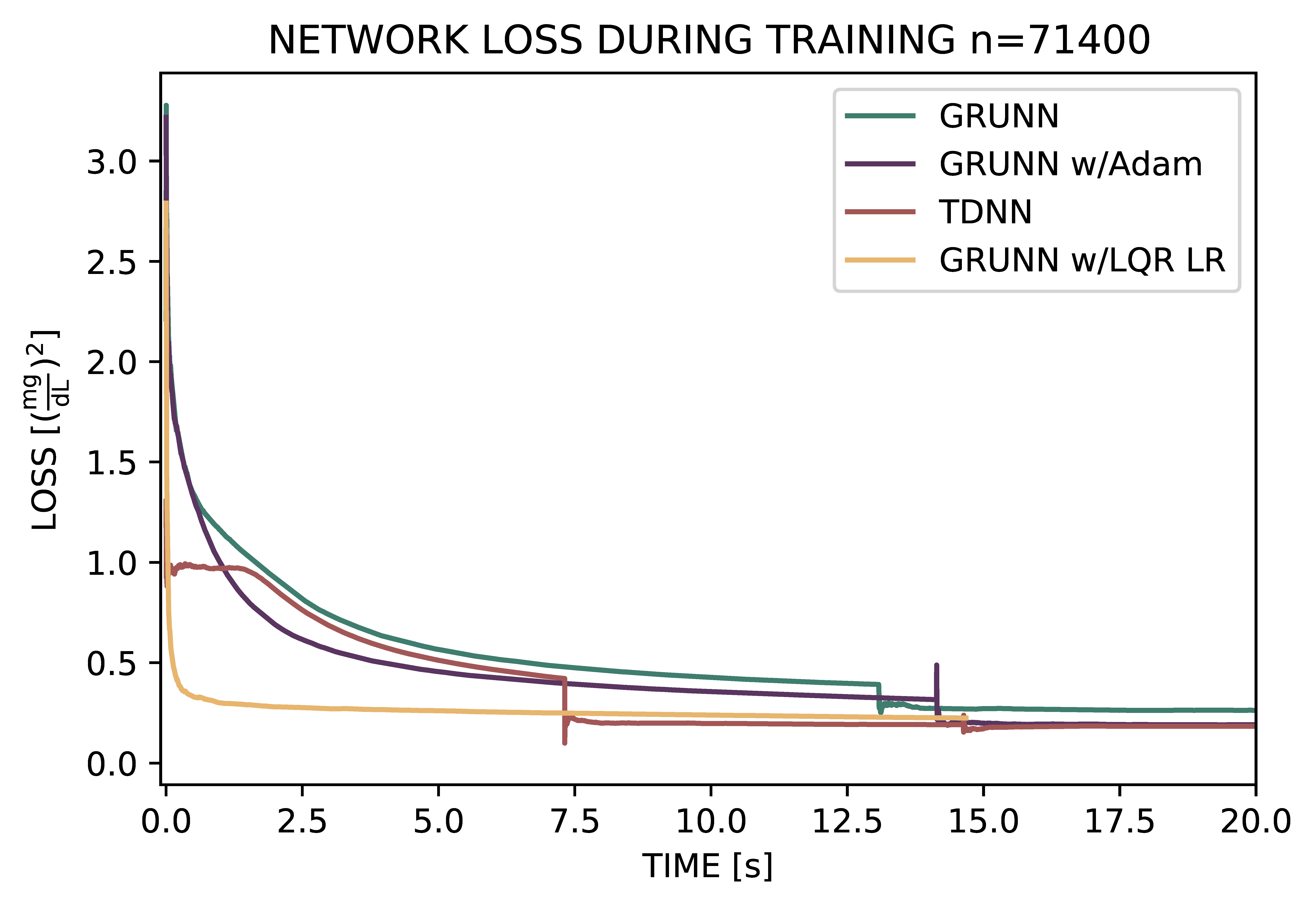
Improving robustness came at a cost: training time increased significantly for the GRU NN over the original design. To reduce the computational overhead, I implemented a novel controller to streamline training using a principle of control system engineering known as LQR control.
In the graph, the GRU NN training is compared to the industry standard method (Adam), the original design (TDNN), and the novel controlled training method (GRUNN w/LQR LR). With the new method, training time and stability was improved over both NN designs. Note that the error (loss) converges to a minimum much faster with the LQR method.
